Author Correction: Reconstitution of Rab- and SNARE-dependent membrane fusion by synthetic endosomes
It was brought to our attention by Elisabeth M. Bik that, in Ohya et al. Nature 2009, the blots indicated by Syntaxin 13 in Fig. 2a are duplicates in lanes 1 and 3 and in lanes 4 and 5. In addition, the two blots indicated by EEA1 and rabenosyn-5 in lane 4 of Fig. 2a are duplicates of the two blots indicated by EEA1 and rabenosyn-5 in Fig. 2b lane 1. We have conducted a thorough internal investigation by reanalysing all original data available in our archive. Out of the eight spotted panels, we found one technical mistake that does not change any conclusions of the paper. Below is the summary of our investigation and the original autoradiographs used to prepare the figures.
To understand Fig. 2a and Fig. 2b, it is important to note the following:
-
1.
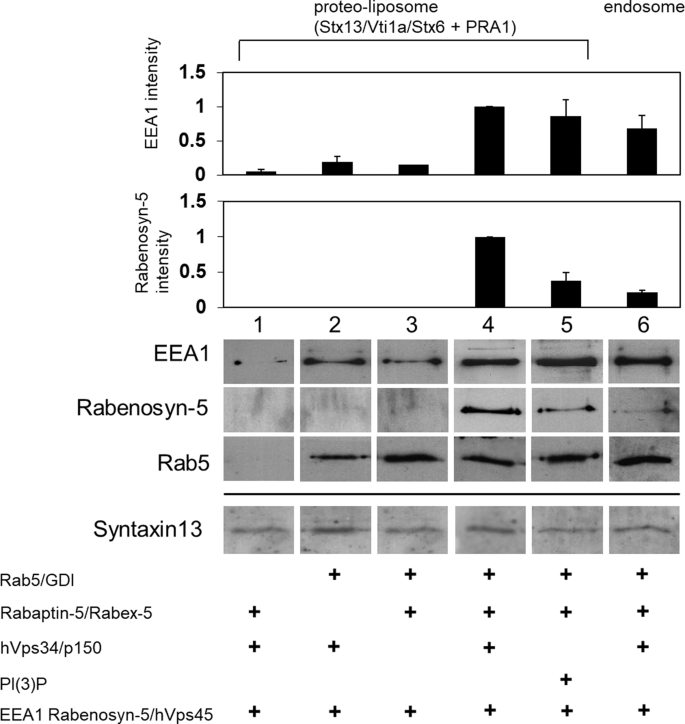
The panels were assembled using data from different experiments with many reactions consisting of different combinations of proteoliposomes containing integral membrane proteins, e.g., Syntaxin 13, VTI1A and Syntaxin 6. The proteoliposomes were split between different tubes and soluble recombinant proteins, such as Rab5, EEA1, rabenosyn-5, etc., were added in different combinations (as indicated) to each reaction.
-
2.
Figures 2a and b show a composite of different experiments, where the panels show images of the proteins in the reconstitution experiments as representative examples. This is because the complexity of the biochemical experiments requires different reactions, internally controlled and reproducible. Several such experiments were performed over a period of two years (2006–2008). There was no selection of experiments, all experimental data were analysed and included in the statistics. An example of the reproducibility of the data is shown below (see Supplementary Information), where two autoradiographies of experiments conducted on 05/01/07 and 11/01/07 are compared side by side.
-
3.
The amount of soluble proteins recruited to the proteoliposomes were estimated by quantitative western blots (WB), as described. Owing to limitations of materials, the WB of Syntaxin13 could be done on the mother mixtures only. Two original Syntaxin 13 WB of the mother mixtures used in Fig. 2a are shown below.
-
a)
The blots of Syntaxin 13 are identical in lanes 1 and 3 because the very same proteoliposomes were used and incubated with soluble proteins as indicated. The WB band of Fig. 2a lanes 1 and 3 corresponds to lane 5 of the original autoradiograph 09/08/08 (see Supplementary Figures).
-
b)
Figure 2a lane 2 corresponds to lane 4 of the same autoradiograph.
-
c)
Figure 2a lane 4 was assembled incorrectly because it corresponds to lane 1 of the original autoradiograph 09/08/08.
-
d)
Figure 2a lane 5 corresponds to lanes 3 of the original autoradiograph 31/08/08 (Supplementary Figures).
-
e)
Figure 2a lane 6 corresponds to lanes 4 of the original autoradiograph 31/08/08
-
4.
Figure 2b compares the recruitment of soluble recombinant proteins by proteoliposomes containing different combinations of transmembrane proteins (Syntaxin 13 vs. Syntaxin 7). Figure 2b lanes 1 and 2 contain the same proteoliposomes and soluble recombinant proteins as in Fig. 2a, lane 4 (complete reaction with proteoliposomes and soluble proteins), except that the reaction is incubated in the absence (Fig. 2b, lane 1) or presence (Fig. 2b, lane 2) of cytosol. This means that the association of soluble proteins (EEA1, rabenosyn-5 as well as Rab5) with the membrane has to be reassessed also for the contribution by cytosol (Fig. 2b, lane 2). The experiment demonstrates that it is the mix of endosomal transmembrane proteins that regulates the membrane recruitment of the soluble proteins, with no significant contribution by cytosol. We identified a small mistake in the drawing of the error bar in Fig. 2a, EEA1 lane 4, which is identical to Fig. 2b, lane 1, and have corrected it.
Most importantly, all proteins in the figures, including Syntaxin13, were normalized by the amount of phospholipids to maintain the protein/phospholipid ratio. This is important because even if the proteins levels are comparable, the protein/phospholipid ratio must be controlled.
We would thank Elisabeth M. Bik for bringing this issue to our attention and providing us with the opportunity to clarify the figures for the Nature readership. We apologize for not having explained that the WB band of Fig. 2a, lanes 1 and 3 were the same; for having inadvertently duplicated the band of Syntaxin 13 in Fig. 2a, lanes 4 and 5; and for not having explained that the Syntaxin 13 WB band of Fig. 2b, lane 1 was the same as in Fig. 2a, lane 4. We have prepared a corrected Fig. 2a (see Fig. 1 below) in which the Syntaxin 13 band of lane 4 has been replaced with the correct one, and the legend has been edited accordingly.
Recruitment of Rab5, EEA1 and rabenosyn-5 on proteoliposomes. a, Proteoliposomes with a similar phospholipid and Syntaxin 13 (STX13) content as early endosomes (as control, lane 6) were incubated with the indicated proteins (see Methods). Membrane-associated proteins were detected by western blotting, quantified and normalized to 1 (arbitrary units, a.u.) with the values in lane 4. The bands shown are representative of at least three repeat experiments. There was no selection of experiments; all experimental data were analysed and included in the statistics. The WB of Syntaxin13 were done on the proteoliposomes mother mixtures split between different reactions and presented for comparison with endogenous levels of Syntaxin13 in early endosomes (lane 6). Note that the band of Syntaxin 13 is identical in lanes 1 and 3 because the very same proteoliposomes were used. b, Cognate t-SNAREs are necessary for the stable membrane recruitment of EEA1 and rabenosyn-5. Proteoliposomes with different SNARE sets were incubated with recombinant proteins with or without further incubation with cytosol. Note that the EEA1 and rabenosyn-5 WB bands of lane 1 in Fig. 2b are the same as those of lane 4 in Fig. 2a, and shown for comparison with the reaction in the presence of cytosol (Fig. 2b, lane 2). Membrane association was measured as in a. c, d, Cognate t-SNAREs are necessary for cytosol-dependent proteoliposome fusion. Membrane fusion of either cognate (lanes 1 and 2) or non-cognate (lanes 3 and 4) t-SNARE proteoliposomes with endosomes (c) or v-SNARE proteoliposomes (d) with or without cytosol was measured as in Fig. 1. Histograms show the average of three independent experiments and s.e.m.
We apologize for the confusion that the lack of detailed information on the figures may have caused.